What Is A Conjugated Pi System
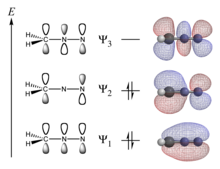
What is a conjugated pi system. They allow a delocalization of pi electrons across all the adjacent aligned p-orbitals. The reaction occurs through a cyclic transition state. Polyene ribbon π-systems are arranged so that the lowest energy ψ1 MOs have p orbital phases which match right along the top and the bottom of the system.
Cumulated double bonds This molecule is known as allene containing two double bonds which share a carbon atom. In a conjugated pi-system electrons are able to capture certain photons as the electrons resonate along a certain distance of p-orbitals - similar to how a radio antenna detects photons along its length. Its a little bit like a pi bond but extended over more than two carbons kind of like a row of men on a foosball table.
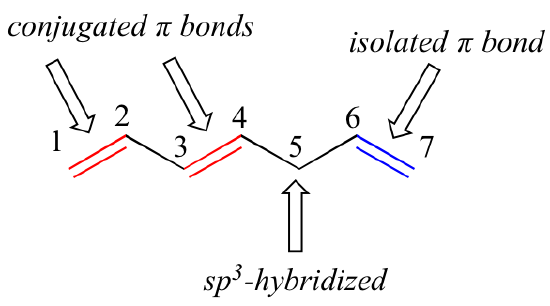
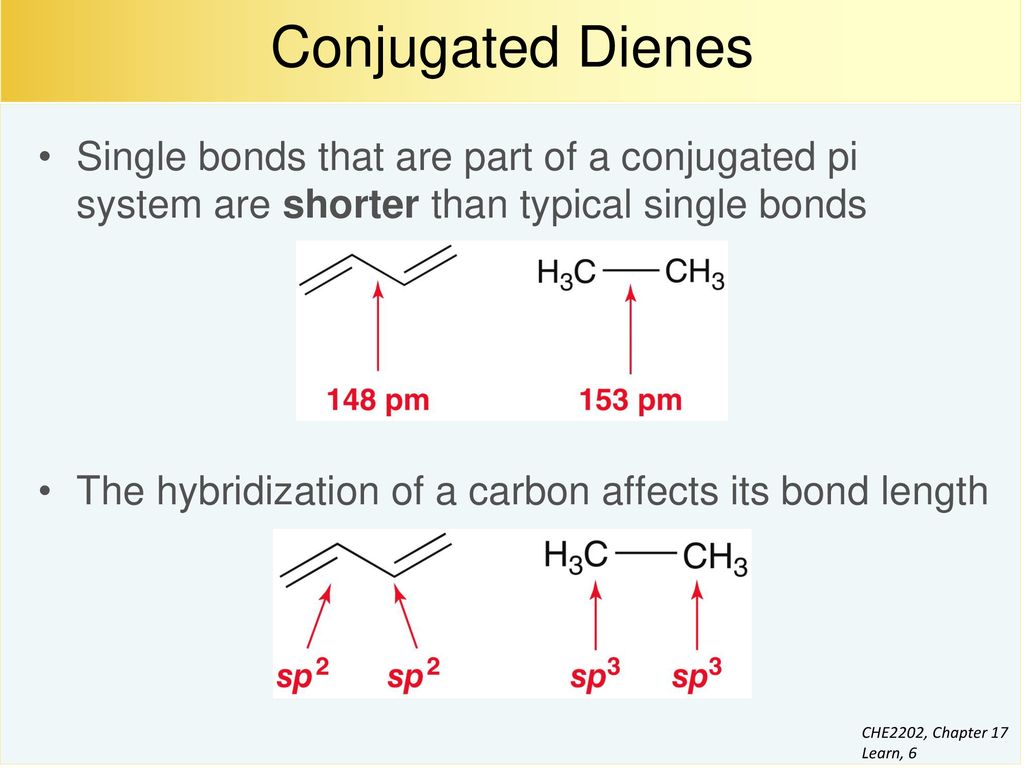
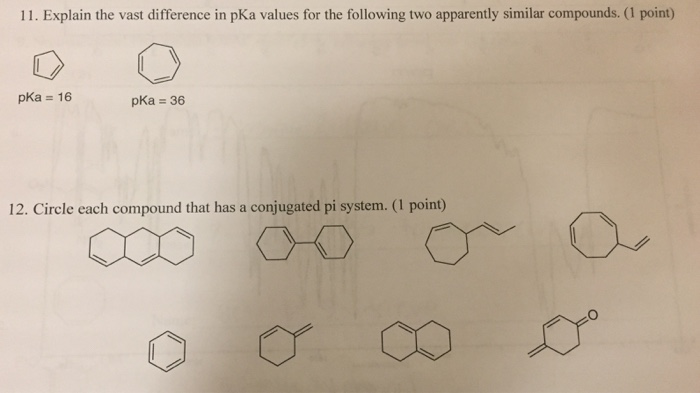
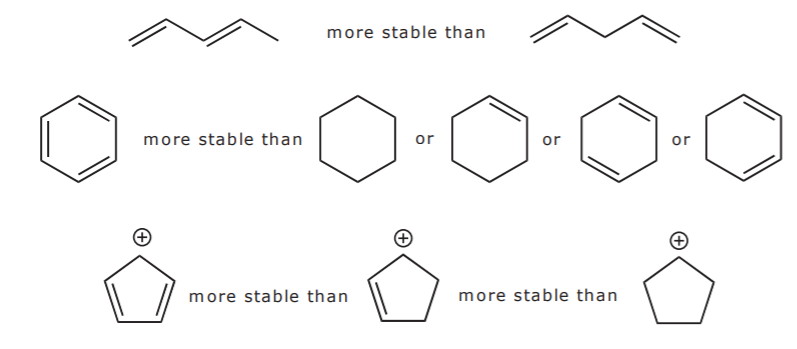
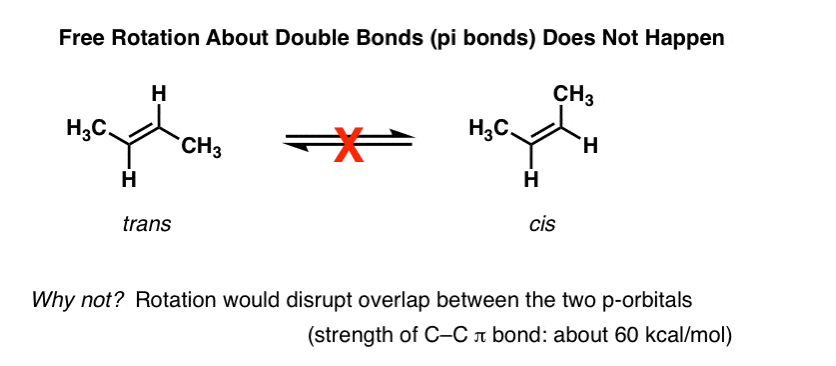
Conjugation is what we call it when 3 or more p orbitals join together into a larger pi system. It can also generate bond rotations but you can bet that the conjugated pi system will remain planar as the energy barrier to rotation about the sp2 carbon bonds is very high. Number of atomic orbitals used to combine.
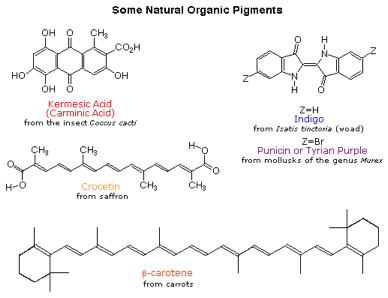
In other words with every added adjacent double bond we see in a molecule diagram we can predict the system will be progressively more likely to appear yellow to our eyes as it is less likely to absorb yellow light and more likely to absorb red light. Of the resultant bond upon addition of the atomic orbitals. The reaction proceeds via a concerted process which means that all changes in bonding occur in a single step.
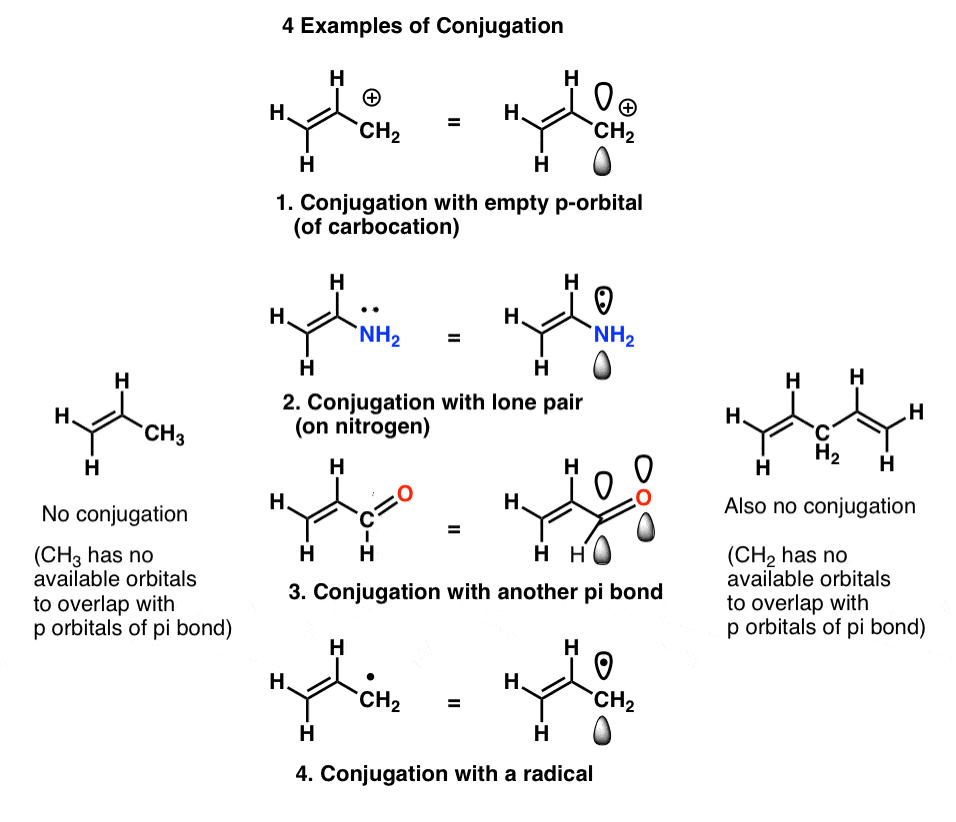
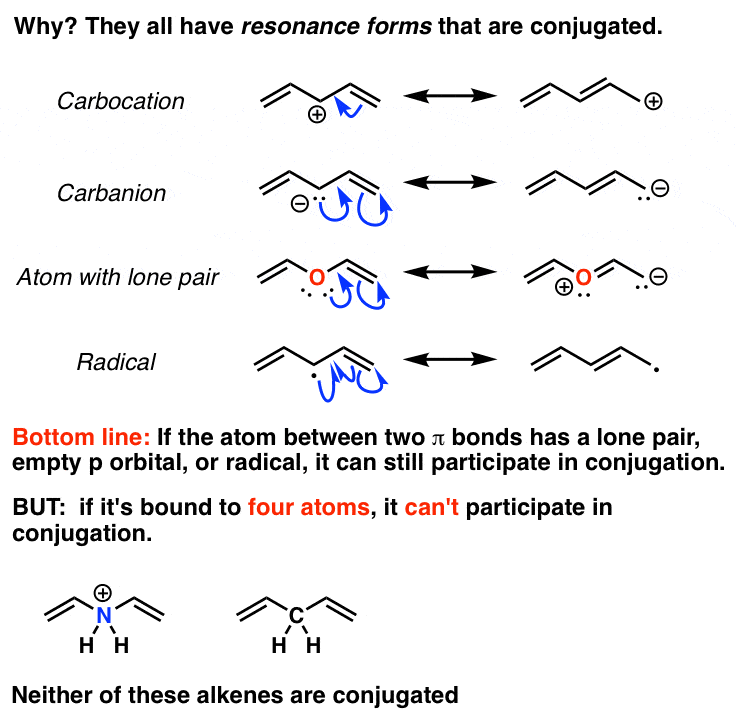
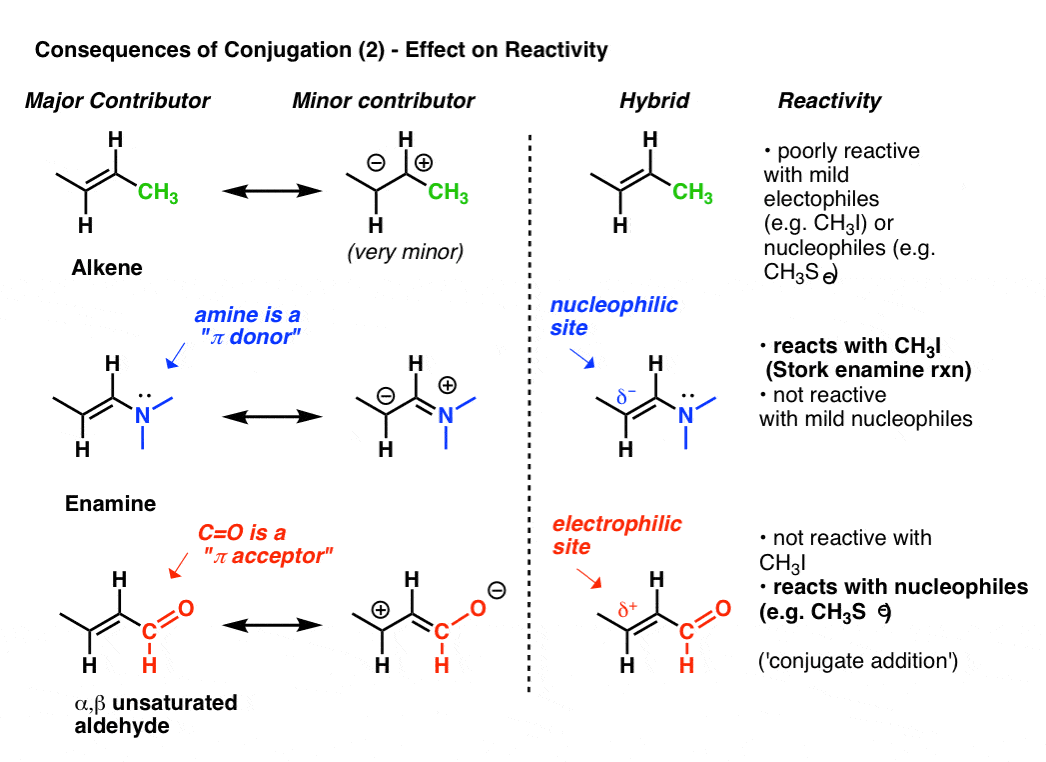
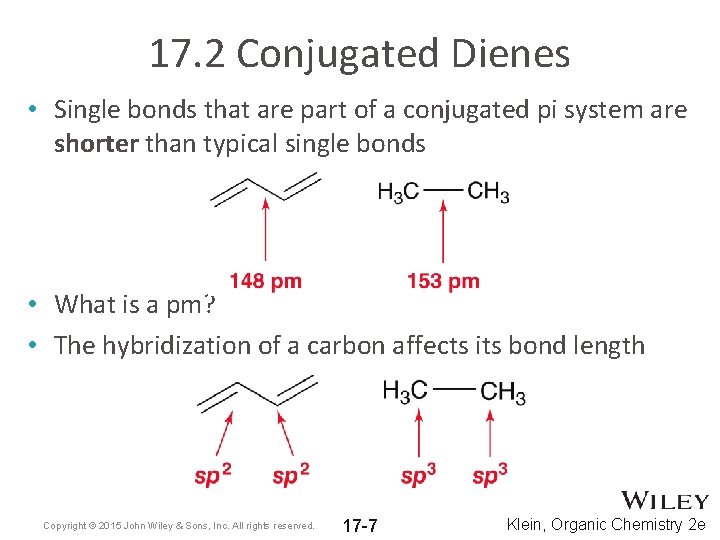
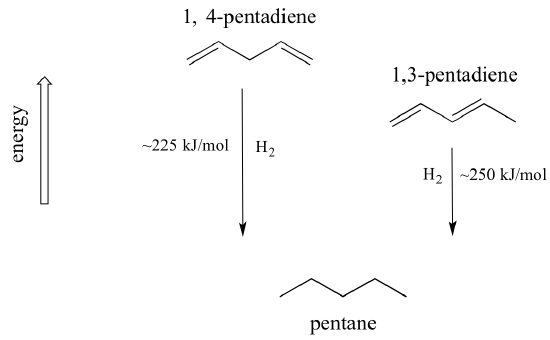
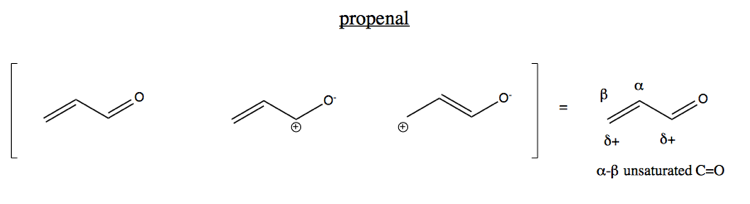
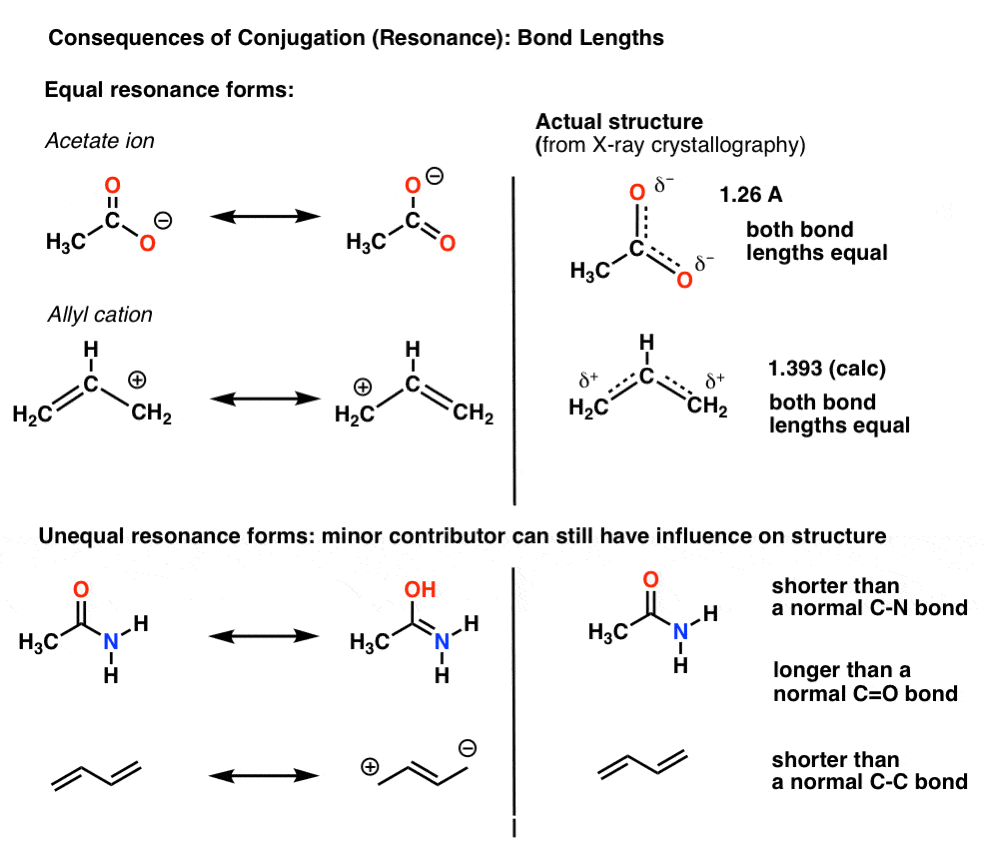
In a normal bond the electrons are localised between the constituent atoms. Organic compounds are almost endless in number and range in size from small molecules to macromolecules. A conjugated system is a system of connected p-orbitals with delocalized electrons in compounds with alternating single and multiple bonds which in general may lower the overall energy of the.
These conjugated pi systems contain electrons which we often call pi electrons to distinguish them from the electrons that comprise single bonds in the molecule. The reaction involves a ring of electrons moving around in a closed loop. 24 Conjugated Pi Bond Systems - Chemistry LibreTexts.
A less highly conjugated system will require the absorption of the higher energy part of the. How do you tell if a system is.
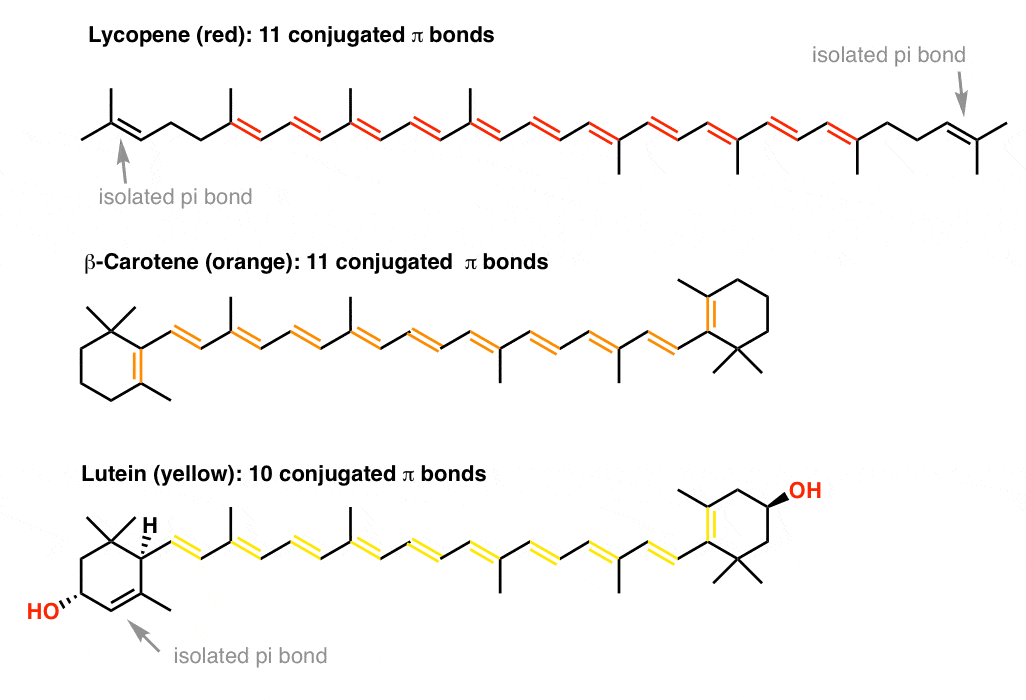
As the polyenes grow in length more electrons are required to retain near 11 electrical neutrality.
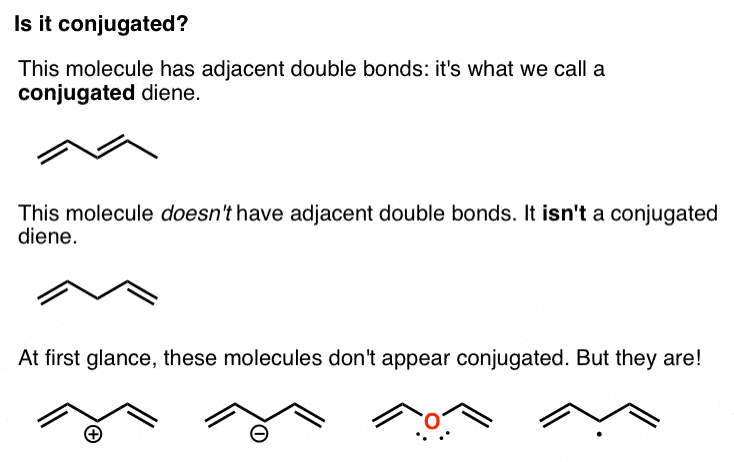
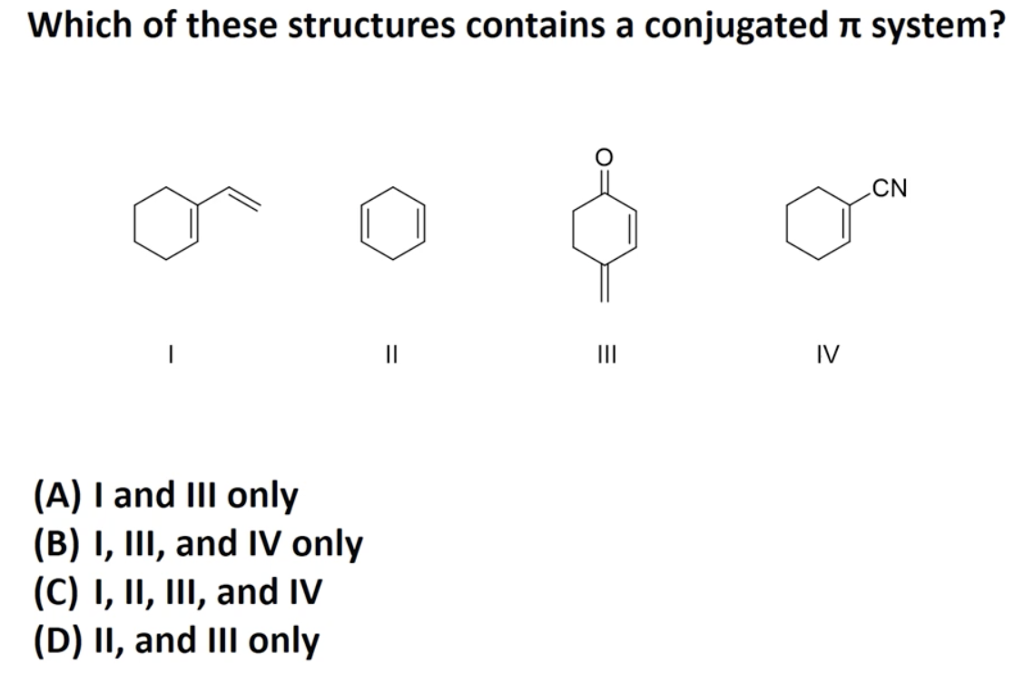
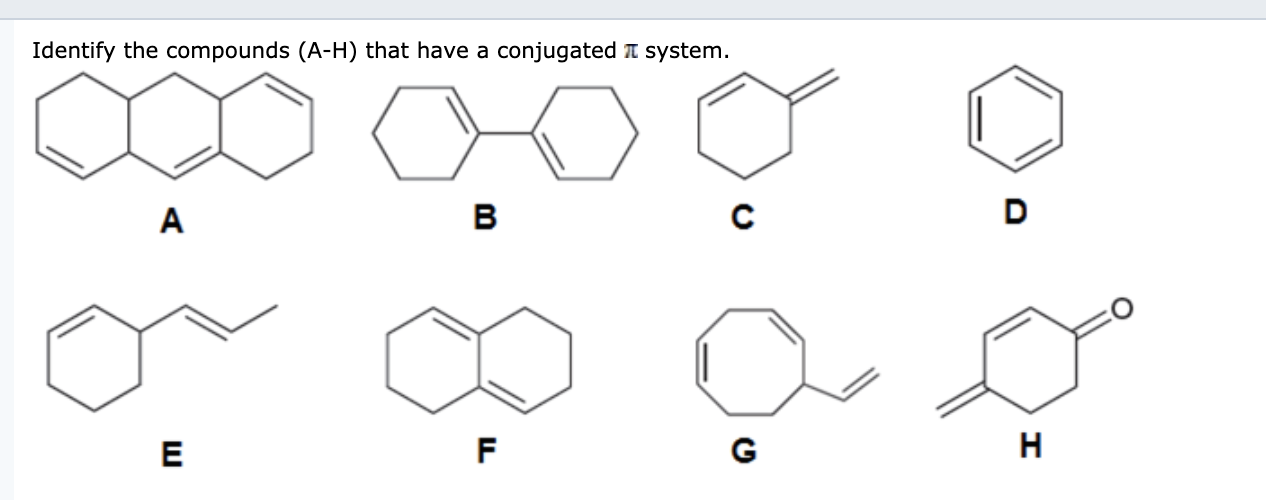
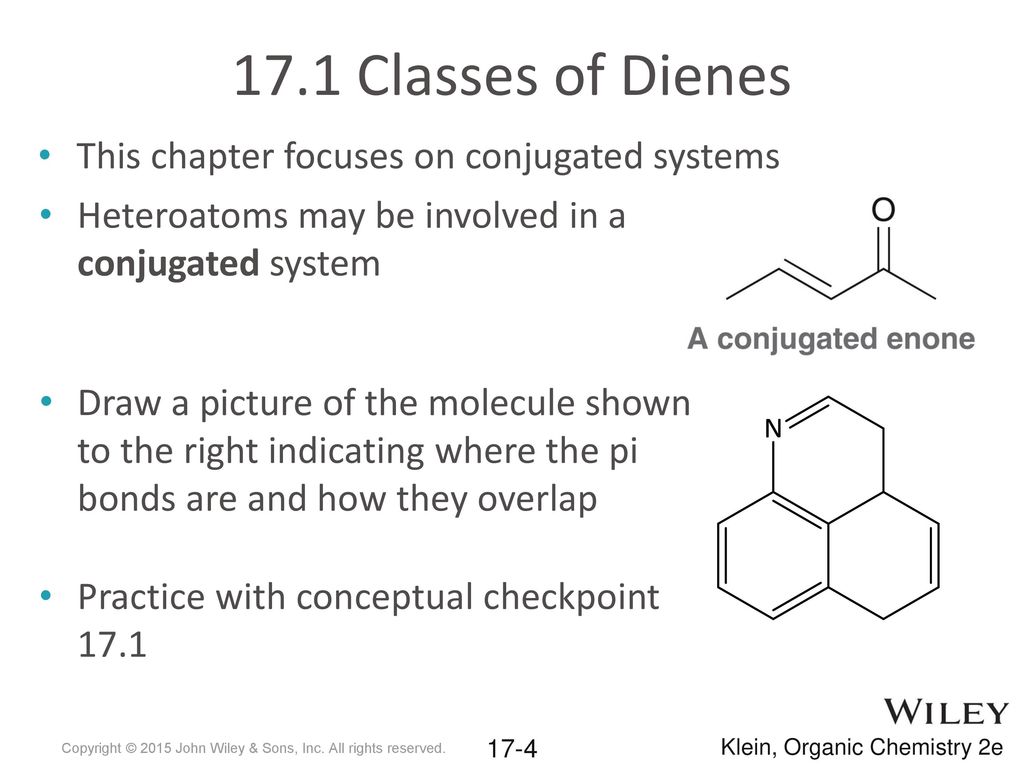
It can also be written as. The reaction involves a ring of electrons moving around in a closed loop. They allow a delocalization of pi electrons. A conjugated system is a system of connected p-orbitals with delocalized electrons in compounds with alternating single and multiple bonds which in general may lower the overall energy of the molecule and increase stability. These conjugated pi systems contain electrons which we often call pi electrons to distinguish them from the electrons that comprise single bonds in the molecule. What is a conjugated double bond. Organic semiconductors are mainly π-conjugated systems which are classified into two groups based on the weight namely π-conjugated polymers and small molecules. Typically the more conjugated longer the pi-system is the longer the wavelength of photon can be captured. Any time theres a group of three or more adjacent p orbitals that can all line up in the same plane this is a conjugated system or pi system.
1 Always get the same number of molecular orbitals as. The electrons in those p orbitals then get delocalized inside this electron cloud. The carbon at the center is abbreviated as a single dot. As the polyenes grow in length more electrons are required to retain near 11 electrical neutrality. They allow a delocalization of pi electrons across all the adjacent aligned p-orbitals. These conjugated pi systems contain electrons which we often call pi electrons to distinguish them from the electrons that comprise single bonds in the molecule. ChemDraw will give you a simple list.






















Post a Comment for "What Is A Conjugated Pi System"